
In the pharmaceutical industry, product quality is determined not only by the formulation but also by the packaging that protects it. Plastic packaging that comes into direct contact with medicines must meet stringent safety standards to prevent contamination that could affect the stability and effectiveness of the final product. For this reason, microbiological testing and Escherichia coli MPN (Most Probable Number) analysis play an essential role in the quality assurance process for pharmaceutical packaging.
Microbiological Testing
Microbiological testing ensures that packaging is free from microorganisms that may be introduced during manufacturing, from molding and cooling to the final packing stage. Although plastic is an inert material, its production process still carries the risk of exposure to bacteria, molds, and yeasts.
International pharmacopeias set specific microbiological limits for packaging materials that come into direct contact with pharmaceutical products. Through this testing, the total microbial count can be measured and evaluated against established safety thresholds. Ensuring that packaging stays within these limits is crucial to preventing secondary contamination of the finished product.
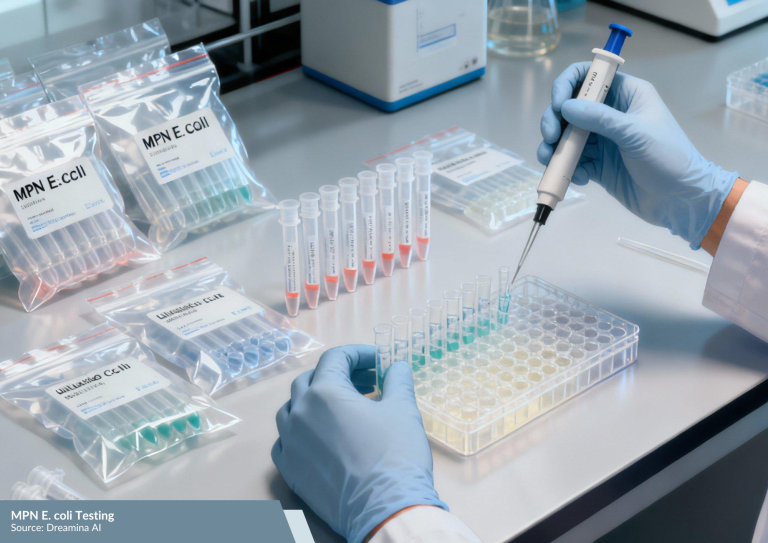
MPN E. coli Testing
In addition to general microbiological analysis, specific testing is carried out to detect the presence of E. coli. This bacterium serves as an indicator of hygiene conditions, as its detection often points to inadequate sanitation in the production environment.
The Most Probable Number (MPN) method is used to estimate the concentration of E. coli in a sample. A “not detected” result indicates that production processes are well-controlled, facility hygiene is maintained, and the packaging is safe to be used as a primary pharmaceutical container.
Benefits for the Pharmaceutical Industry
Conducting microbiological and MPN E. coli testing provides multiple advantages, including:
- Ensuring packaging is safe and suitable for pharmaceutical applications
- Strengthening customer confidence through verifiable quality data
- Reducing the risk of product defects or potential recalls
- Supporting compliance with audits, ISO certifications, and regulatory requirements such as those set by the National Agency of Drug and Food Control (BPOM)
Medion Plastic’s Commitment to Quality and Safety
These testing practices reflect the company’s commitment to delivering high-quality plastic packaging. Beyond laboratory analysis, maintaining hygiene throughout the production chain is vital to consistently meeting defined quality standards.
Through strict process controls and comprehensive testing, Medion Plastic ensures that every packaging product delivered supports the safety and integrity of pharmaceutical products.
